Liquid phase solvation dynamics
Solvation dynamics of elementary charge carrier in polar solvents:
Liquid water, the native medium for biochemical and cellular processes, consists of a complex network of polar molecules connected by hydrogen bonds. Water responds to the presence of a solute, like protons, eltrons or ions, by changing its local structure and dynamics on a multitude of length and time scales.
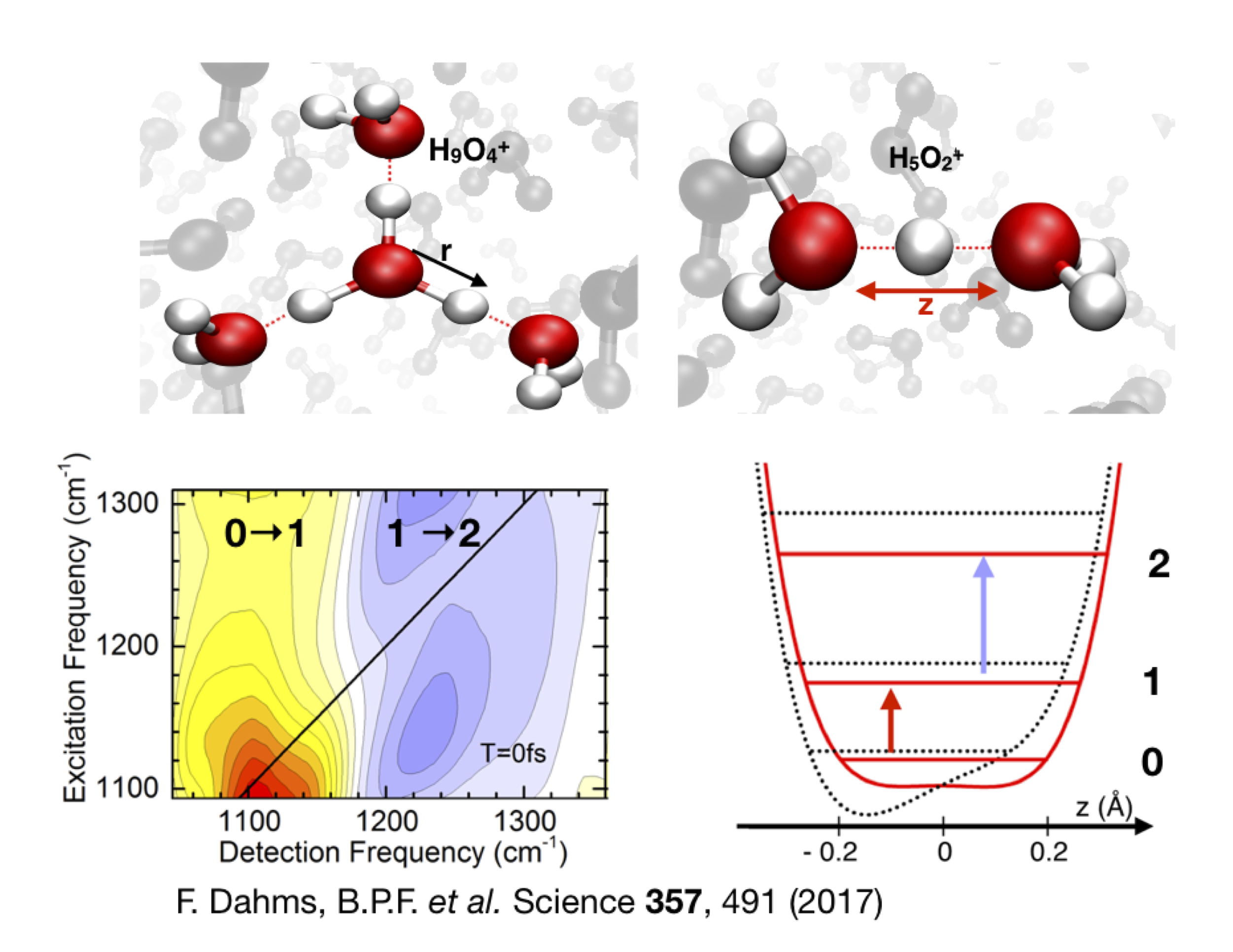
Water makes the proton shake – ultrafast motions and fleeting geometries in proton hydration:
Basic processes in chemistry and biology involve protons in a water environment but water structures accommodating protons and their motions have largely remained elusive. Applying ultrafast vibrational spectroscopy and simultions, we have mapped fluctuating proton transfer motions. The reuslts provide direct evidence that protons in liquid water are predominantly shared by two water molecules. Femtosecond proton elongations within a hydration site are 10 to 50 times faster than proton hopping to a new site, the elementary proton transfer step in chemistry [1],[2].
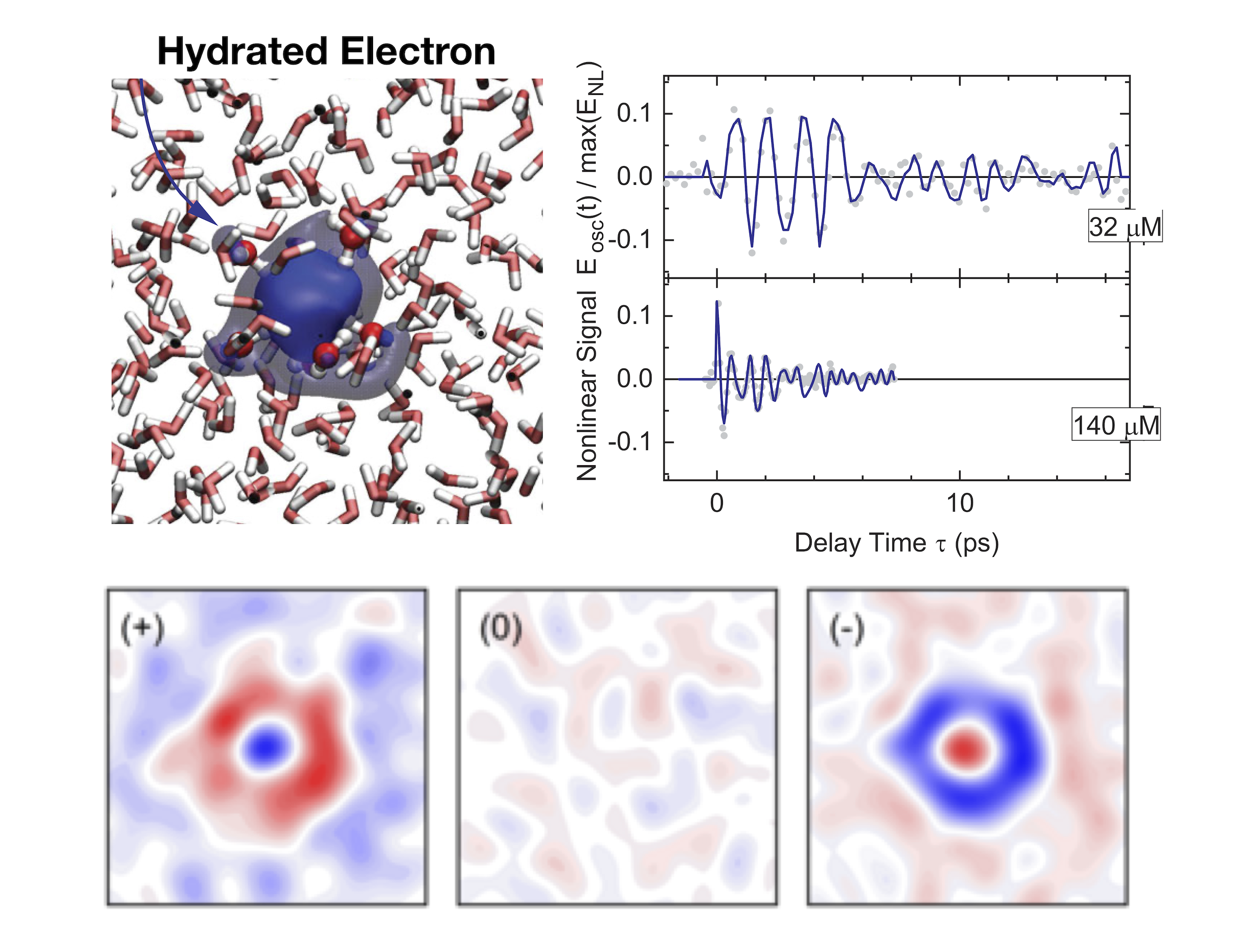
Terahertz waves from electrons oscillating in polar solvents:
Ionization of water molecules by light generates free electrons in liquid water. After generation, the so-called solvated electron is formed, a localized electron surrounded by a shell of water molecules. In the ultrafast localization process, the electron and its water shell display strong oscillations, giving rise to terahertz emission for tens of picoseconds [3],[4].
Magnesium ions slow down the water dynamics on short length scales:
The influence of negatively and positively charged ions on liquid water is usually classified via the Hofmeister series which ranks ions based on their ability to structure the water around them or to disrupt the water structure. The microscopic origin and molecular mechanisms of the Hoffmeister series are controversial, despite many years of research. Recent experiments and simulations have now revealed a significantly more complex influence of ions on the dynamics of surrounding water molecules. We used the asymmetric stretching vibrations of sulfate (SO42-) ions as locally sensitive probes to map the dynamic properties of the environment. We found that the presence of Mg2+ ions reduces the ultrafast fluctuations of the water shell around a sulfate ion, leading to a specific slowdown in the solvation dynamics of hydrated MgSO4. [5]
Key Publications:
-
F. Dahms, B. P. Fingerhut, E. T. J. Nibbering, E. Pines, T. Elsaesser, “Large-amplitude transfer motion of hydrated excess protons mapped by ultrafast 2D IR spectroscopy.” Science 357, 491-495 (2017).
-
A. Kundu, F. Dahms, B. P. Fingerhut, E. T. J. Nibbering, E. Pines, Thomas E., "Hydrated Excess Protons in Acetonitrile/Water Mixtures – Solvation Species and Ultrafast Proton Motions." J. Phys. Chem Lett. 10, 2287-2294.
-
A. Ghalgaoui, B. P. Fingerhut, K. Reimann, T. Elsaesser, M. Woerner, “Terahertz Polaron Oscillations of Electrons Solvated in Liquid Water.” Physical Review Letters 126, 097401 (2021).
-
P. Singh, J. Zhang, A. Ghalgaoui, K. Reimann, B. P Fingerhut, M. Woerner, T. Elsaesser, “Coherent polaron dynamics of electrons solvated in polar liquids.” PNAS Nexus 1, pgac078 (2022).
-
A. Kundu, S. I. Mamatkulov, F. N. Brünig, D. J. Bonthuis, R. R. Netz, T. Elsaesser, B. P. Fingerhut, “Short-Range Cooperative Slow-down of Water Solvation Dynamics Around SO42– – Mg2+ Ion Pairs” ACS Phys. Chem Au 2, 506–514 (2022).